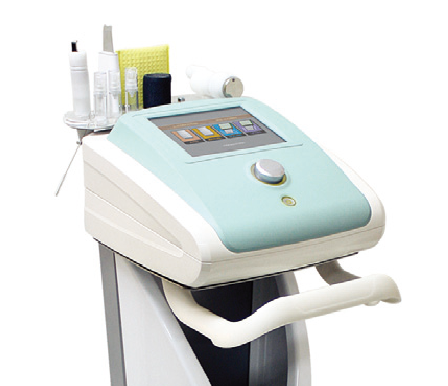
MESOLEX
CWM-530
Low Frequency stimulator
Notice
This product is certified as a medical device in the European Union under the medical Device directive 93/42/EEC by SGS CE0120,
exclusively for the intended purpose of muscle pain relief. Other non-medical uses ascribed to this device are not within the scope of CE certification,
and users should be aware product performance and/or safety has not been evaluated by SGS for those purpose.
| Output freguency | 500~6000Hz |
|---|---|
| Duty rate | 5~90% |
| Intensity | 0~50Level |
| Mode | Positive&Negative |
| Time | 1~60mins adjustable |
| Temperature | 0~-10℃ / 37~42℃ |
| Dimension(mm) | 330(W) X 354.5(D) X 187(H) |
| Weight | 8kg |
| Power | AC 220V,50/60HZ |
| Power consumption | 110VA |
